INTERVENTIONAL PRODUCTS

Breakthrough products for peripheral interventional treatment
In an era of chronic healthcare workforce shortages and a growing burden of care, new medical devices should enable improved efficacy and productivity of care while minimizing complexity of use. Breakthroughs are needed—not simply incremental improvements.
This is the spirit that guides our development of peripheral interventional products.
With a combined vessel diameter range of 2–10mm, the Pounce™ Thrombectomy Platform provides a standalone solution for rapid removal of peripheral arterial thrombi and emboli throughout the lower and upper extremities.
Pounce™ Thrombectomy Platform
2–10mm

COMBINED VESSEL DIAMETER RANGE
2–4mm
3.5–6mm
5.5–10mm
Rapid removal of acute or chronic thrombi or emboli without thrombolysis
NO aspiration used for clot removal–minimizes blood loss
NO capital equipment
Grab, go,
restore flow
SFA angiography before (A)
and after (B) 2 passes of the Pounce System, with thromboembolic debris
removed after initial pass (C).*
Proprietary dual-basket design.
Clot removed after initial pass of the Pounce™ Thrombectomy System*
- Bacharach MJ, Bacharach T. Successful Removal of 20 cm SFA Thrombus With the Pounce™ Thrombectomy System. Endovascular Today. 2022;21(12 Suppl):S12-13.
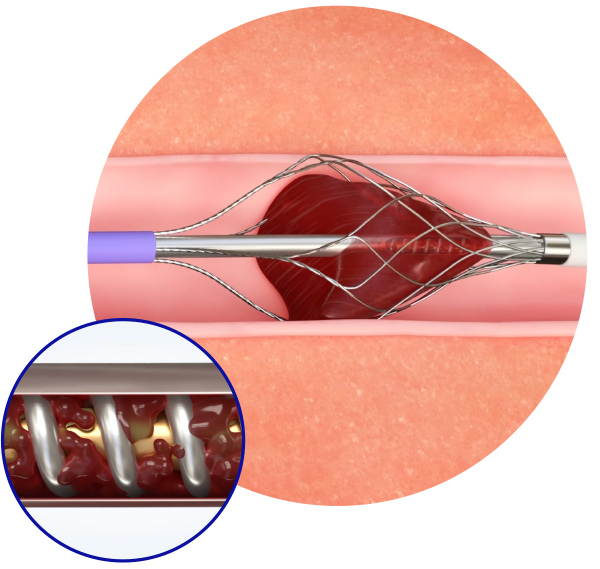
Pounce™ Venous Thrombectomy System
To remove iliofemoral venous clots, most interventionalists choose between suction or mechanical devices. The Pounce™ Venous Thrombectomy system uses a dynamic, fully collapsible basket to remove wall-adherent clot while extracting the clot from within the basket.
Visit pouncevenous.com
10 Fr
Pounce™ Venous Thrombectomy System
Dual-action technology capable of removing wall-adherent thrombus in a single session without the need for thrombolytics.
“Wow, that’s a clean vein.”
Dr. Brandon Repko
Interventional Radiologist
Butler Memorial Hospital
Butler, PA
Fem-pop venous thrombus before (left) and after (right) treatment with the Pounce™ Venous Thrombectomy System
Dr. Repko is not a consultant for Surmodics.
Sublime™ Radial Access Platform
Radial access reduces complications and patient stays while improving patient satisfaction.1,2 The Sublime™ radial access platform makes radial-to-peripheral treatment of peripheral artery disease (PAD) practical, not just possible with a full suite of radial-purposed tools designed to deliver robust kink resistance, torque, and crossability.3
Visit sublimeradial.com
Go wrist to foot—with confidence.
Crossing the pedal loop and up the contralateral tibial artery with the 250 cm Sublime™ Radial Access .014” RX PTA Dilatation Catheter
References
- Mason PJ, Shah B, Tamis-Holland JE, et al. An update on radial artery access and best practices for transradial coronary angiography and intervention in acute coronary syndrome: a scientific statement from the American Heart Association. Circ Cardiovasc Interv. 2018;11(9):e000035.
- Cooper CJ, El-Shiekh RA, Cohen DJ, et al. Effect of transradial access on quality of life and cost of cardiac catheterization: a randomized comparison. Am Heart J. 1999;138(3):430-436.
- Data on file at Surmodics, Inc.
Caution: Federal (US) law restricts the Pounce™ Thrombectomy System, the Pounce™ LP Thrombectomy System, the Pounce™ Venous Thrombectomy System, and the Sublime™ Radial Access Platform to sale by or on the order of a physician. Please refer to Instructions for Use for indications, contraindications, warnings, and precautions.